2019 Conference
 Image credit: iStock
Image credit: iStock
Details
Major themes addressed at the conference were Shiny, reproducible research, package administration, scaling R for production, and using R in a regulatory environment.
This post was originally shared on R/Views.
It’s no secret that there are few industries more competitive than the pharmaceutical industry. Big money placed on long-shot bets for block-buster drugs where being first makes all the difference means a constant struggle to gain a competitive edge. So, you might find it surprising that the inaugural R / Pharma Conference held this past August on the Harvard campus in a very classy auditorium was all about collaboration.
Some might also find it surprising that data scientists from competitive companies would gather to share information, but this is quite common. I have seen it before in other competitive industries, for example in IEEE-led standards initiatives, where engineers gather to forge a common technology. Not only is there the human need to share and learn from peers (and also brag a little), there is a larger force at play: a kind of market clearing operation where experts gather to gain as much of an advantage as they can by ensuring that no easily exploitable arbitrage opportunities remain.
It was a surprise, though (and I think a source of general amusement as the conference proceeded), that nearly every talk seemed to be about Shiny. Looking back, it is clear that it should not have been: 49% of the abstracts explicitly mention Shiny. This word cloud was built from the abstract submissions.
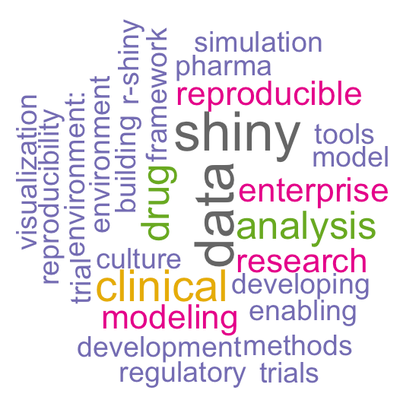
Shiny is basically a technology for sharing complex information across multiple organizations and stakeholders with different skill sets. Shiny, too, is all about collaboration. For a look into the large, production-grade Shiny app, bioWARP, see Sebastian Wolf’s recent post.
Other major themes addressed at the conference were: reproducible research, package administration, scaling R for production, and using R in a regulatory environment. This last theme was underscored by a strong FDA presence. Lilliam Rosario from the FDA Center for Drug Evaluation & Research delivered the opening keynote, in which she addressed the regulatory role of CDER and the use of R. FDA speaker Mat Souktup spoke about the need to transcend the compartmentalized culture common in medical research, and how open-source tools are helpful in working towards this goal. He explicitly noted along the way that the FDA does not specify what software may be used. The third FDA speaker, Paul Schuette, filled in some details associated with topics raised by Rosario and talked about the use of R and Shiny at CDER. Along these same lines, Andy Nicholls from GSK conducted a well-attended and very informative workshop on The Challenges of Validating R. You can find Andy’s slides here.
Other keynote speakers were Max Kuhn, who talked about Modeling in the tidyverse (slides here); Joe Cheng, who described how to use Shiny responsibly in pharma (slides here); and Michael Lawrence, who spoke about enabling open-source analytics in the enterprise.
My very biased impression was that R / Pharma was an unqualified success at accomplishing the major objectives of bringing together data scientists and statisticians working in the Pharmaceutical industry, and of presenting a high quality program that explored several issues relating to the production use of R in a regulatory environment.
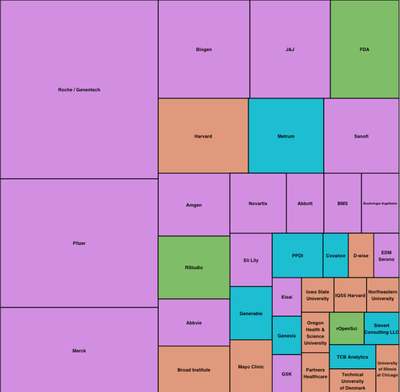
The following chart shows that representatives from quite a few pharmaceutical companies attended in spite of organization problems that artificially limited the overall number of attendees to about 140.
R/Pharma 2019 schedule
All times below in US ET.
-
8:30 AM
-
8:40 AM
-
8:40 AM
Artificial neural networks in R with Keras and TensorFlow
Leon Eyrich Jessen, Technical University of Denmark
-
1:30 PM
-
1:30 PM
-
1:30 PM
-
8:15 AM
Package Management (Coffee session)
-
8:15 AM
R Education in Pharma (Coffee session)
-
8:15 AM
R and Python Interoperability (Coffee session)
-
8:45 AM
Coffee
-
9:00 AM
Opening Remarks
-
9:15 AM
-
10:00 AM
-
10:20 AM
-
10:30 AM
-
10:50 AM
Coffee
-
11:10 AM
-
11:30 AM
-
11:40 AM
Exploratory Graphics (xGx): Promoting the purposeful exploration of PKPD data
Alison Margolskee, Novartis
-
12:00 PM
-
12:10 PM
Using RStudio.Cloud to advance R proficiency: a crowdsourcing training experience
Paulo Bargo, Janssen
-
12:30 PM
Lunch
-
1:30 PM
-
1:50 PM
R Packages for Analyzing Clinical Trials Data with R Focusing on Safety And Early Efficacy
Nina Qi, Roche / Genentech
-
2:00 PM
-
2:20 PM
-
2:30 PM
Collaborating at scale: managing an enterprise analytical computing ecosystem
Rena Yang, Roche / Genentech
-
2:50 PM
Embrace R in Pharma - building internal R community and establishing fit-for-purpose R pilots
Ning Leng, Roche / Genentech
-
3:00 PM
-
3:20 PM
-
3:30 PM
Coffee
-
3:50 PM
-
4:35 PM
Leveraging multiple R tools to make effective pediatric dosing decisions
Jeannine Fisher, Metrum Research Group
-
4:45 PM
Using Machine Learning and Interactive Graphics to Find New Cancer Targets
David Cooper, Glaxosmithkline
-
5:05 PM
-
5:15 PM
An R package for Data Science and Deep Visualization of a complex clinical database
David James, Novartis
-
8:15 AM
Shiny for Early Drug Discovery Research (Coffee session)
-
8:15 AM
**** (Coffee session)
-
8:15 AM
Shiny in Production (Coffee session)
-
8:45 AM
Break
-
9:00 AM
Opening Remarks
-
9:05 AM
-
9:45 AM
Your Missing Step in Reproducible R Programming: Continuous Deployment
Chase Clark, University of Illinois
-
10:00 AM
-
10:20 AM
-
10:30 AM
Break
-
10:50 AM
-
11:10 AM
-
11:30 AM
-
11:40 AM
Evaluating the performance of advanced causal inference methods applied to healthcare claims data
Jessica Myers Franklin, Harvard University
-
12:00 PM
Leon Eyrich Jessen, Technical University of Denmark
-
12:10 PM
Lunch
-
12:30 PM
-
1:30 PM
Jason Stevens, Bristol Myers Squibb
-
1:50 PM
-
2:00 PM
-
2:20 PM
Coffee
-
2:30 PM
Using R to foster the communication with non-statisticians on Bayesian dose escalation models
Marianna Grinberg, Merck
-
2:40 PM
Madeleine S. Gastonguay, Metrum Research Group
-
3:00 PM
-
3:10 PM
From CDISC to TLFs, using R to support Pharmacokinetic Analyses
Jessica Higgins, Nuventra Pharma Sciences
-
3:30 PM
Coffee
-
3:40 PM
-
4:00 PM
-
4:45 PM
-
4:55 PM
Debbie Morreall, University of Utah
-
5:15 PM